
Microtubule-associated protein 1A/1B-light chain 3 (LC3) is a soluble protein with a molecular mass of ∼17 kDa that is distributed ubiquitously in mammalian tissues and cultured cells. The error has been corrected in all versions of the article. *Note: In the version of the article initially published online, the words “Gel buffer (3x)” were missing in the table on page 18. Here I describe a protocol for Tricine–SDS-PAGE, which includes efficient methods for Coomassie blue or silver staining and electroblotting, thereby increasing the versatility of the approach. Tricine–SDS-PAGE is also used preferentially for doubled SDS-PAGE (dSDS-PAGE), a proteomic tool used to isolate extremely hydrophobic proteins for mass spectrometric identification, and it offers advantages for resolution of the second dimension after blue-native PAGE (BN-PAGE) and clear-native PAGE (CN-PAGE). These lower concentrations facilitate electroblotting, which is particularly crucial for hydrophobic proteins. The concentrations of acrylamide used in the gels are lower than in other electrophoretic systems.

It is the preferred electrophoretic system for the resolution of proteins smaller than 30 kDa. Tween is a registered trademark of ICI Americas.Tricine–SDS-PAGE is commonly used to separate proteins in the mass range 1–100 kDa. Note: high salt can also reduce signal strength of the target protein. Increasing the salt concentration in all buffers to 0.5M NaCl will reduce background. View our recommended Western Blot Buffer Groups.If the intensity of the target band is still too low, but background is not a problem, 1% BSA can be used as the blocking component. Adjust concentration of milk up or down to obtain desired signal strength and low background. Start with 5% nonfat dry milk for block, and 2% nonfat dry milk for primary and secondary antibody dilution. Adjust up or down to obtain desired signal strength and low background. (This is typically equivalent to 15-30 μg of total protein). 10-20 μL of cell lysates at 1x10 7 cells per mL. Adjust antibody concentration from 0.05 up to 2.0 μg/mL to obtain desired signal strength and low background. Optimization may be done as an initial checkerboard screen where multiple conditions are applied in a single experiment or sequentially, changing one set of parameters at a time and optimizing conditions over several blots. The variables with the most significant impact are listed below. The objective in optimizing Western blotting conditions is to maximize signal strength and minimize non-specific bands and background noise.

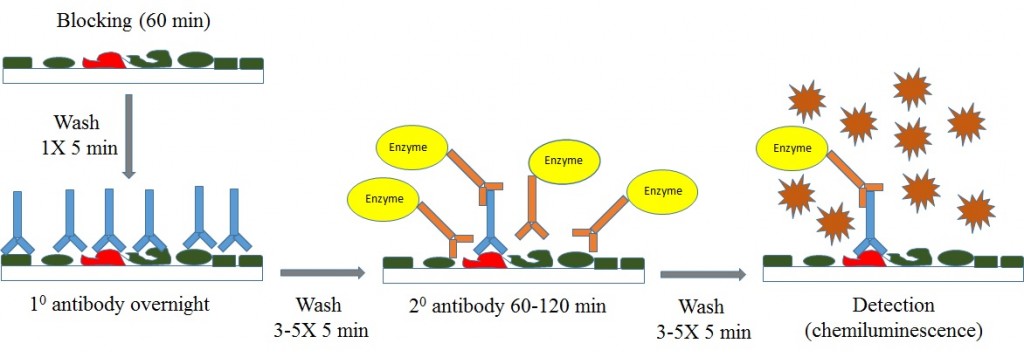
When running Western blot with cell lysates, multiple parameters may need to be optimized for antibody detection of endogenous protein levels.


 0 kommentar(er)
0 kommentar(er)
